ORIGINAL RESEARCH |
https://doi.org/10.5005/jp-journals-10048-0065 |
Preparation Ability of ProTaper Next and XP-endo Shaper Instruments in Isthmus-containing Root Canal System
1,2 Department of Endodontics, Faculty of Dentistry, Gazi University, Ankara, Turkey
Corresponding Author: Tayfun Alaçam, Department of Endodontics, Faculty of Dentistry, Gazi University, Ankara, Turkey, Phone: +90 5522117677, e-mail: talacam@gazi.edu.tr
How to cite this article: Sarıkahya M, Alaçam T. Preparation Ability of ProTaper Next and XP-endo Shaper Instruments in Isthmus-containing Root Canal System. Cons Dent Endod J 2020;5(2):28–35.
Source of support: The work was supported by Projects of Scientific Investigation of Gazi University, Ankara, Turkey, with code no: 03/2017-10.
Conflict of interest: None
ABSTRACT
Aim and objective: This research compares the abilities of the preparation of ProTaper Next (PTN; Dentsply-Maillefer) and XP-endo Shaper (XPS; FKG, Switzerland) files in mesial canals of lower molars with separate binary canals and one foramen with isthmus connection using μCT imaging.
Materials and methods: The comparison showed 20 roots matched according to the similarities in preoperative canal volume, specimen length, and root curvature using preoperative scans, and then they were indiscriminately separated into two groups and prepared either with PTN or XPS instruments. After chemomechanical preparation, the roots were resubmitted to postoperative scans.
Results: There was no variance in instrument systems for the volume of removed dentine, surface area as well as the amount of accumulated debris in the isthmus region (p >0.05). Change in canal surface area and amount of accumulated hard tissue debris (AHTD) was more in the PTN group for total root canal space (p <0.05). PTN instruments increased surface area in a shorter period. Conclusion: Both instrument systems with different design and metallurgic properties had reached the desired volume in different periods; however, neither technique was able to fully prepare the isthmus-containing mesial roots of lower molars.
Keywords: Isthmus cleaning, M-wire, and Max-wire, NiTi instruments, Rotary instruments, Shaping ability.
INTRODUCTION
It could be valuable for the success of endodontic procedure to choose a particular system or protocol that influences the ability to shape and clean main canals and the locations that have irregularities like the isthmus region.1,2 The intrinsic design and functional limitations of the endodontic instruments mostly lead to insufficient shaping of the canals.3
It is quite a challenging phase to clean all regions, including isthmuses and fins during endodontic therapy, despite the advancements in instrument technology and instrumentation methods. Nevertheless, this respect resulted in the progress of a new generation of metals, including novel NiTi M-wire and max-wire instruments.
ProTaper Next (PTN) files (Dentsply-Mailleffer), manufactured with M-wire technology, have an increasingly percentage-tapered design on each file. M-wires receive further thermomechanical treatment, and their nano-crystalline martensitic microstructure presents mechanical assets that can extract files further elastic and resistant to fatigue than those prepared with usually handled NiTi wires.4 PTN files create exclusive unique rotary movements, and, at any particular cross-section, it only contacts the canal surface in two areas.5,6 This system decrease engagement as a result of its swaggering outcome, which limits unwanted taper lock.7 It gives extra cross-sectional volume for better preparation and removing the debris and also lets every PTN cut a better envelope of motion related to a like-sized instrument with asymmetrical form and circumrotation.8,9 However, when PTN compared with Twisted File system, they presented similar untouched canal surface areas and accumulated hard tissue debris (AHTD) in isthmus-containing mesial roots of mandibular molars and both systems produced a suboptimal and similar mechanical preparation in one study.10 In another study, Linden et al.11 mechanically prepared mesial canals of lower molars using PTN X3 applying sonic, ultrasonic, and syringe final irrigation. All tested supplementary irrigation steps significantly reduced the amount of debris created during root canal preparation. Stringheta et al.12 compared the shaping ability of PTN, Reciproc, WaveOne Gold, and ProDesign Logic in curved molar root canals using microCT. There was no significant difference among the instrumentation systems regarding the volume of dentin removed, an increase in root canal volume, untouched root canal surface, and volume of AHTD.
XP-endo Shaper (XPS; FKG Dentairie, Switzerland) instruments respond to variations in temperature also seize on a programmed form within the canal at body temperature. Max-wire is a metallurgical structure that gives the instrument high flexibility.13 Its snake-shaped form offers the file an extra-flexible nature even at a fast speed (i.e., 850 rates per minute). The XPS expands further than the original core size to adjust to the root canal.14 It is found that fewer smears in an apical area by using XPS with conventional irrigation compared to iRaCe with SEM evaluation.15 Elnaghy et al.16 studied to assess the efficacy of the XP-endo Finisher (FKG Dentaire SA, La Chaux-de-Fonds, Switzerland) file on debris and smear layer removal in curved root canals in comparison to different irrigation regimens. The mesial root canals were mechanically prepared using the BT-Race rotary system (FKG Dentaire) and divided into five groups according to the following irrigation techniques: positive control, nonagitated, file agitation, XP-endo Finisher, and EndoActivator (EA) (Dentsply Tulsa Dental Specialties, Tulsa, OK, USA). Irrigation of curved root canals using XP-endo Finisher and EA methods appears to be more effective on debris and smear layer removal than the other tested groups. Zhao et al.17 evaluated the outcome of preparation with Reciproc Blue with XPS systems in C-shaped canals, and then both files were left with similar amount unprepared canal surface area and Reciproc Blue (RB) left considerably more levels of AHTD in comparison with XPS. Leoni et al.18 evaluated the possibility of using the XP Finisher instrument in the mechanical finishing of root canal preparation with isthmuses and demonstrated the reduction of accumulated debris after biomechanical preparation. Jayakumaar et al.19 evaluated the efficacy of XP-endo Finisher with PTN and HyFlex in smear layer and debris removal. Lower debris and smear layer scores were seen in canal thirds instrumented with PTN and XP-endo Finisher. Similarly, Alakshar et al.20 assessed and compared XP-endo Finisher cleaning efficiency concerning the amount of remaining debris and smear layer vs Max-I-Probe needle (CI), EA device, and combination of XP-endo Finisher file with EA device (XP + EA). They concluded that EA and CI showed less debris and smear layer than XP and XP + EA in the middle and apical third. The use of the XP conjunction with 17% ethylenediaminetetraacetic acid (EDTA) and 2.5% NaOCl irrigation failed to have a debris-free dentin surface in the apical portion of root canals.
Other early in vitro studies have revealed encouraging results with max-wire XP-Finisher instrument on the matter of the elimination of bacteria, AHTD, canal obturation, and Ca(OH)2 paste from the canals.18,21–24 However, further surveys are essential to establish the capability of these novel files for cleaning and shaping the root canals for different variations of canal anatomy.
Mesial roots of mandibular molars with isthmuses have been selected for the evaluation of the performance of instrumentation in a plethora of studies;10,11,18 however, lower molar teeth present a high degree of complexity, not allowing a realistic evaluation of file systems true capability and performance level, reducing the possibility to achieve optimal results in terms of shaping ability and AHTD. So, there is a need for more study which reduced root canal configuration biases that might interfere with the results.
In this context, the drive of this research is to contrast the success of PTN and XPS instruments for the performance of the instrument in root canal shaping, which includes the proportion of unprepared surface areas and accumulated debris amounts in mesial canals of lower molars with Vertucci type II formation (Vertucci, 1984)25 both in total canal space and in isthmus region.
MATERIALS AND METHODS
Sample Size Calculation
In the beginning, a power analysis was made to create the number of specimens in study groups. The effect size was d = 1.4, α = 0.05, 1 - β = 0.84 Group = 2 n1 = n2 = 10.
Nsum = 20; and hence with 95% safety and 1.4 effect size, theoretical power value was obtained as 84%.
Inclusion/Exclusion Standards
The selected teeth were lower molars with two disconnected canals and one foramen with isthmus connection in mesial roots. The subsequent elimination standards were carried out: (a) roots with attached, fractured, cracked, atypical roots or teeth that had earlier root canal treatment; and (b) roots had above 20° of curvature.26 Both buccolingual and mesiodistal parallel radiographs were obtained for each tooth to evaluate the morphology. Roots with another configuration type except class II were left out.25 After a preliminary inspection, 160 mesial roots of newly extracted lower molar teeth were chosen for the study. The roots were kept in 0.1% thymol liquid until the beginning of the experiment.
Cone-beam Computed Tomography Scans for Sample Selection
The next step was to separate crowns and distal roots of lower molar teeth and to embed mesial roots in silicone molds. Cone-beam Computed Tomography scans were taken at all levels from the apex using the One-Volume viewer program (J Morita Mfg) for the selection of Vertucci type II canal system with the isthmus. At this point, 26 roots met the selection criteria.
Preoperative μCT Scans
Afterward, mesial roots were attached to a special tray and scanned individually by a μCT device (SkyScan 1172, Bruker μCT). All scanning procedures were performed at 100 kV source voltage with 100 μA source current and 360° spin through a 0.05° rotation step, ensuing in an image with a 10.03 μm pixel size. This is made by using a copper and aluminum filter.
Six more teeth were discarded for abnormal canal configurations after the first capture of images. Teeth were collected by the likenesses in μCT scans for preoperative canal volume, and sample size along with the root curvature. Thus, 20 roots were selected (Flowchart 1).
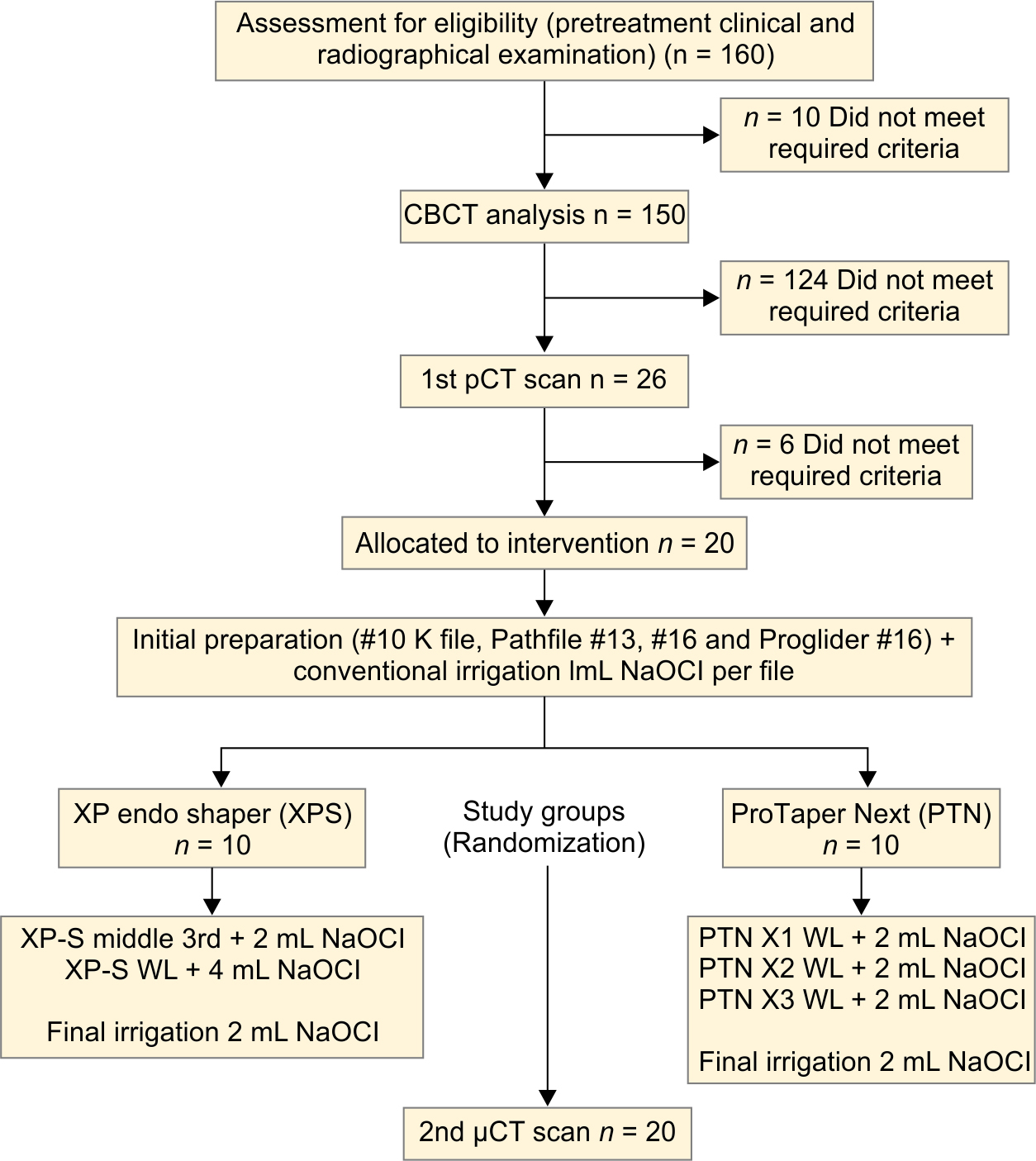
Flowchart 1: Flow diagram of the study
Root Canal Preparations
Canal patencies were confirmed with a K-file. After the file tip was observable through the main foramen, 0.5 mm draw off to define working length (WL). At that time, the apex of every specimen was closed by epoxy resin to make a locked end method. A glide path was achieved to WL using both Pathfile (#13, #16) and ProGlider (#16) instruments (Dentsply-Mailleffer). The idea was to distribute samples casually into two groups. The first group of 10 teeth was instrumented with PTN instruments and the second group of 10 teeth with XP-endo Shaper instruments. It was established to use parameters for PTN and XPS instruments according to manufacturer's guidelines, which are 850 rpm with 1 Ncm of torque for XPS and 300 rpm 2 Ncm of torque for PTN files. XPS group was prepared by using only a single file (#30, 4%). After dipping to 37°C water bath, the XPS instrument initially was used in one-third of the canal with up and down motion five times. Then XPS instrument was used with the same motion at WL for five strokes with repeated three treatment applications. PTN group was prepared with the files X1 (#17, 4% at WL), X2 (#25, 6% at WL), and X3 (#30, 7% at WL). X1 and X2 instruments were used with five strokes in up and down motions with the crown down method. On the other hand, the X3 instrument was used ten times for equal application of stroke and time with the XPS instrument. A fresh set of files used to perform with each canal preparation. Throughout each preparation cycle, 2 mL of 5.25% NaOCl at 37°C was used with the aid of a Max-I-Probe (Dentsply Maileffer) irrigation needle to 4 mm coronal of WL for both instrumentation groups.
After each irrigation regiment, push–pull movements of gutta-percha cones were done in full length by hand at an estimated frequency of 100 strokes for 1 minute in each canal. The abundance of the measures of XPS group controlled by using #30, 4% gutta-percha (FKG Dentaire), and the same controls applied with X3 gutta-percha (Dentsply-Maillefer) for PTN group. Thus each canal was irrigated with a total 12 mL of NaOCl solution (1 mL NaOCl after #10 K file, Pathfile #13, #16, and Proglider #16) +6 mL after every preparation and 2 mL with final irrigation). By the end of the irrigation procedure, canals final washing was carried out with sterilized saline and dehydrated by several paper-points.
Preparations were achieved via one endodontist who has 6 years of experience and is blinded to the three-dimensional (3D) simulated specimens.
A standardized manual fashion was run for the applications in a torque testing platform using X-smart IQ endomotor. To achieve more precise torque settings, real-time torque monitoring (RTTM(tm) Technology) was run by using Endo IQ iOS app for iPad (Dentsply Sirona). Before treatment, calibration function (CAL) was used for both instrument systems for more precise torque settings. Total mechanical instrumentation time was recorded for both instrument systems.
Postoperative μCT Scan and Evaluation Methodology
After the biomechanical preparation, the roots once again go through a second μCT scanning with the first parameter settings. Data recreated into transverse-section slices (NRecon v1.6.5.2; Skyscan) with a ray hardening adjustment of 15%, smoothing of five, ring artifact correction of seven, and an attenuation coefficient ranging from 0.00007 to 0.025, resulting in the acquisition of 1,400 to 1,500 transverse cross-sections per specimen, giving axial transverse sections of the interior structure of the specimen. The volume of interest was chosen, expanding from the bifurcation region to the apex. Preoperative/postoperative 3D simulations of the mesial canals were extracted (CTVol v.2.2.1; Bruker-microCT) and co-registered with its own original sets, by rigid registering segment within 3D Slicer 4.6.2 software (https://download.slicer.org/). Later on, paired data were inspected to analyze volume (in mm3) and surface area (in mm2) of the mesial root canal system, preimaging/postimaging with CTAn v.1.14.4 software (Bruker microCT). Also, 2D and 3D volumetric inquiry and measurements of surface area were made with the same software. A difference of every parameter was measured through the difference of results for prepreparation/postpreparation.
CTVol program was acquired for 3D imaging and qualitative assessment of the preinstrumentation/postinstrumentation procedure. Color-coded simulations allowed a qualitative assessment of the paired root canals prepreparation and postpreparation.
The scans were transferred to the Fiji platform (Fiji v.1.47n; Madison, WI, USA) and were regularized for numerical investigation of AHTD. The series of data generating from this process were further used to categorize the debris through morphologic procedures. Quantifying the debris was done through differences amid uninstrumented and instrumented canal volume with postprocessing operations. The occurrence of a substance with a density like dentine in areas formerly filled with space in the unprepared root canal region was reflected as debris and measured by intersectioning the images precanal and postcanal preparation.18,27–30 The entire volume of debris is measured in mm3 unit and stated as the ratio of the root canal and root canal isthmus volume of debris postinstrumentation (vol%).
Statistical Analysis
Volume and canal surface area evaluations for the isthmus area were made by the Mann–Whitney U test. Preoperative–postoperative difference values were used for evaluations of total canal space (Δ). As for the normality assumption, Kolmogorov Smirnov and Shapiro Wilk tests were adopted. t-test was used for the analysis of normally and independently distributed data (canal surface and volume difference). In independent groups without the normality assumption, the Mann–Whitney test was applied.
RESULTS
Evaluation for Isthmus Region
Surface Area and Volume Difference
Both files increased the volume and surface area of this region. No variance was found among the systems (Table 1, Mann–Whitney U test).
| Median | Za | p | |||||
|---|---|---|---|---|---|---|---|
| Instrument | Pre | Post | Pre | Post | Pre | Post | |
| Surface area (mm2) | XPS | 277,100 | 301,300 | −0.91 | −1.28 | 0.393 | 0.218 |
| PTN | 296,200 | 333,650 | |||||
| Volume (mm3) | XPS | 9,650 | 23,900 | −1.21 | −0.53 | 0.247 | 0.631 |
| PTN | 16,650 | 24,400 | |||||
AHTD Evaluation
No variance was found for the isthmus region at 95% safety level (Table 2, Mann–Whitney U test). However, there was an alteration for 90% safety level, and measurements were less for the XPS group.
| Instrument | N | Mean | SD | Test | p | |
|---|---|---|---|---|---|---|
| Total volume, mm3 | XPS | 10 | 41,870 | 14,965 | −1.414b | 0.174 |
| PTN | 10 | 53,620 | 21,596 | |||
| Total surface area, mm2 | XPS | 10 | 395,440 | 227,539 | −2.877b | 0.010 |
| PTN | 10 | 835,990 | 427,539 |
μCT image of mesial and distal views of representative 3D reconstructions of the mesial root canal system of mandibular molar was presented for XPS in Figure 1 and PTN in Figure 2. A sample of the cross-sectional image of the two systems was given in Figure 3.
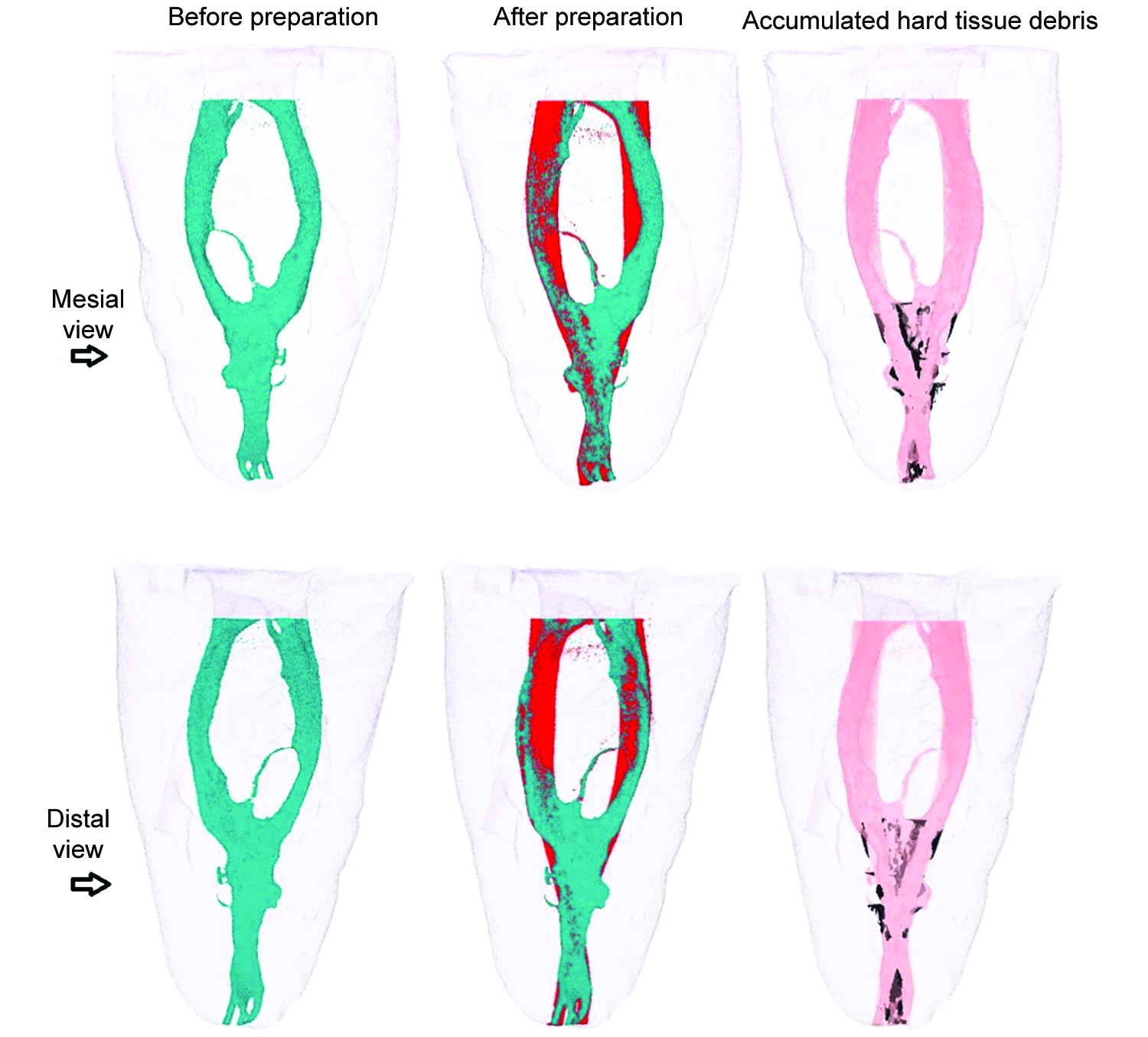
Fig. 1: Mesial and distal views of representative 3D reconstructions of the mesial root canal system of a mandibular molar before (in green) and after (in red) preparation with XP-endo Shaper instruments. Accumulated hard tissue debris (AHTD) is depicted in black.

Fig. 2: Mesial and distal views of representative 3D reconstructions of the mesial root canal system of a mandibular molar before (in green) and after (in red) preparation with PTN instruments. Accumulated hard tissue debris (AHTD) is depicted in black.

Fig. 3: A cross-sectional image of before and after instrumentation
Evaluation of Total Canal Space
Surface Area and Volume Difference
Preoperative and postoperative touched surface differences were statistically significant for groups, and instrumented surface area was higher in PTN instruments (Table 4; t-test, p <0.01). The removed dentine volume difference was not significant for instruments for total canal space (Table 2; t-test).
| Instrument | N | Mean | SD | Test | p | |
|---|---|---|---|---|---|---|
| AHTD in isthmus, mm3 | XPS | 10 | 0.0566 | 0.0469 | −1.89a | 0.059 |
| PTN | 10 | 0.1832 | 0.2071 | |||
| AHTD in total canal, mm3 | XPS | 10 | 0.0228 | 0.0150 | −3.025a | 0.002 |
| PTN | 10 | 0.0460 | 0.0184 |
| XPS | PTN | |
|---|---|---|
| Average | 290 | 226 |
| SD | 54,913 | 44,402 |
AHTD Evaluation
There was an altercation between groups, and the remaining AHTD levels were more in the PTN group (Table 3; Mann Whitney U).
The total mechanical instrumentation time was given in Table 4.
DISCUSSION
The study selected two different rotary instrument systems: a PTN and an XPS for the evaluation of their shaping ability of complex root canals due to their promoted metallurgical properties and geometrical difference. PTN instrument is designed with M-wire, having higher elasticity and apparent smaller Young modulus than that of conventionally treated NiTi files.4 Its superelastic behavior can be dominant for the preparation of complex root canal anatomy, as flexibility conserves the tooth structure, decreases the risk of unintentional mistakes, and eventually lets irrigants to stream more profound in the canals.31,32 XPS files are manufactured with max-wire alloy in which it keeps it in the martensitic stage at room temperature, later it does a stage change at body temperature. Within the austenitic stage, the instrument is specified with the occurrence of the last 10 mm of the working part of the snake-like curl, which lets it infiltrate bigger canal space than conventional files. With the fragile and elastic properties of the instrument with spinning force, it might originate a motion that constantly gives the operational part dissimilar routes which would allow it to come in touch with the extra canal surface. As the difficulty of the canal anatomy makes preparation and controlling of canals difficult throughout root canal treatment, isthmus-containing type II mesial root canals of lower molars was selected for this paper to relate the preparation capability of PTN and XPS systems. A search for more efficient debridement for this complex morphology was the motive of the study.
Evaluation of different instrumentation techniques on dentin debris accumulation could be a valuable approach for the removal strategy of AHTD. This ex vivo study showed the inability of PTN and XPS instruments to clean the root canal space and isthmus region entirely and on the whole touch to the surface area. XPS left less amount of AHTD for total canal space, and this may be due to its dentin cutting process and composition of created debris. This created debris that is more like a microdebris form (FKG Dentaire, 2017).33 This material might have been removed more easily and efficiently. The higher rotation speed of the XPS instrument and its lower taper might generate more turbulence inside the canal, and this could be another reason for less debris finding compared to a larger tapered instrument. The number of the used instrument for preparation may also explain the generation of more AHTD in PTN group. It has been believed that the use of greater tapers could allow more apical placement of irrigant.31 Despite its 0.02 to 0.04 taper, the XPS instrument enabled an equal amount of removed dentine volume and more efficient removal of debris from total canal space compared to PTN instruments. Both instruments were incapable of eliminating all AHTD from the narrow isthmus region in this study. It is relevant to remind that AHTD exclusion was much more problematic in the narrow isthmi areas than the main canals during the instrumentation. This shows that isthmus region planning and debridement are possibly uttered with anatomic structure than the variance in instrument preparation method and materials. These findings highlight the limited ability of endodontic instruments to clean the root canal and reinforce the importance of antibacterial irrigation for enhanced disinfection of the canal system. In these situations, irrigant solutions may assume fundamental importance in biomechanical preparation. Among these solutions advised for biomechanical preparation, sodium hypochlorite is the most used due to its properties. The use of NaOCl followed by EDTA has been recommended as final irrigants. NaOCl acts on organic components of the smear layer and EDTA, calcium-chelating agent, removes the inorganic components of the smear layer effectively and reduces the AHTD from root canals.34,35 For the measurement of the real cleaning efficacy of both instrument systems in this study, the use of EDTA was not preferred for the irrigation of specimens to prevent its activity for clearing away debris.
For predictable treatment outcomes, accumulated debris must be removed from the root canal system. Debris removal is mainly performed through irrigation. Several different systems of mechanical activation of irrigants to improve endodontic disinfection were analyzed for successful cleaning: manual agitation with gutta-percha cones, endodontic instruments or special brushes, vibrating systems activated by low-speed hand-pieces or by sonic or subsonic energy, use of ultrasonic or laser energy to mechanically activate the irrigants and apical negative pressure irrigation systems.36 Recent studies have investigated the efficacy of these techniques in removing AHTD from isthmus-containing mesial root canals of mandibular molars.13,18,30,37,38 In this study, manual agitation with gutta-percha was used for more effective irrigation. Chemomechanical preparation of the root canal through a combination of mechanical instrumentation with XPS and PTN and antibacterial irrigation findings highlight the limited ability of these instruments to clean the root canal and reinforce the importance of effective irrigation for enhanced cleaning of the root canal system. Predictable removal of debris from the complex root canal system remains an elusive goal. Further research is needed to strive for complete debridement of the root canal system.
The penetration depth of the irrigation needle is another factor that can influence the effectiveness of cleaning the root canal system. Inserting needles short of WL during irrigation might prevent debris extrusion,39 so in this study, NaOCl was used with the aid of Max-I-Probe (Dentsply Maileffer) irrigation needle to 4 mm coronal of WL for both instrumentation groups. However, gutta-percha agitation of irrigant was done in full length.
Increasing canal surface area is very important to characterize the completeness of root canal preparation.32 Many kinds of research have revealed untouched areas in the canals, regardless of the preparation method or endodontic scheme used for the preparation.40,41 Both preparation techniques left unprepared surfaces. The complexity of the canal space in this research may have prevented the instruments from acting efficiently on all canal walls and the isthmus region. Variable taper and asymmetrical rectangular cross-section of the PTN instrument enable the file to contact the wall at two points at any given cross-section,9 and this feature might have made the instrument touch more surface area than XPS instruments in total canal space. It is acknowledged that cross-section alters the torsional performance of files. The asymmetrical structure may persuade other forms of forces and torque due to the uneven contact of the instrument with the dentin. The taper dissimilarities give the instrument to maximize cutting effectiveness. Although Azim et al.14 have pointed out that XPS instruments may instrumentate and touch extra canal walls in oval canals concerning VB, because of its snake-like form that enlarges and contracts to adjust to the canal system, this function may have been less efficient in this study, possibly due to restricted areas of complex root canal anatomy of selected roots.
The potential limitation of this study could be a result of relatively small sample size; however, this is common to other microCT studies.13,14
A change of instrumentation and irrigation techniques and combined use of new methods and materials should be considered for locations that have irregularities. Approximation by using suitable files provides more significant interaction with the canal walls, which improves the cleaning capacity and consequently leading to a good prognosis. File systems used for this study succeeded in reaching the aimed dimensions of the endodontic cavity in different periods without any complication; however, it showed an inability to touch all the surfaces of dentinal surfaces and removal of dentinal debris with the use of a traditional irrigation regime.
CONCLUSION
Both instrument systems with different design and metallurgic properties had reached the desired volume in different periods; however, both systems could not provide debris-free canals and able to touch the whole surface.
CLINICAL SIGNIFICANCE
Mandibular molars are known to have anatomical variations of their root canals which are a challenge to treat endodontically, and both instrument systems made of different metallurgical and design properties have remained unable for totally cleaning the mesial root canal system in an attempt to overcome the limitation imposed by the canal configuration. The present results underline less than the ideal ability of current available root canal instrumentation technology to completely debride and clean the root canal space. Endodontists should take into account the morphology of the radicular pulp space and should look for the instrument system and the method may incorporate more than the others.
ACKNOWLEDGMENT
We express our sincere gratitude to Professor Bulent Altunkaynak for the statistical analysis he did for us.
REFERENCES
1. Siqueira JF, Araújo MC, Garcia PF, et al. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod 1997;23(8):499–502. DOI: 10.1016/S0099-2399(97)80309-3.
2. Susin L, Liu Y, Yoon JC, et al. Canal and isthmus debridement efficacies of two irrigant agitation techniques in a closed system. Int Endod J 2010;43(12):1077–1090. DOI: 10.1111/j.1365-2591.2010.01778.x.
3. Bergenholtz G, SpÅngberg L. Controversies in endodontics. Crit Rev Oral Biol Med 2004;15(2):99–114. DOI: 10.1177/154411130401500204.
4. Pereira ESJ, Gomes RO, Leroy AMF, et al. Mechanical behavior of M-Wire and conventional NiTi wire used to manufacture rotary endodontic instruments. Dent Mater 2013;29(12):e318–e324. DOI: 10.1016/j.dental.2013.10.004.
5. Elnaghy AM. Cyclic fatigue resistance of ProTaper Next nickel-titanium rotary files. Int Endod J 2014;47(11):1034–1039. DOI: 10.1111/iej.12244.
6. Zhao D, Shen Y, Peng B, et al. Root canal preparation of mandibular molars with 3 nickel-titanium rotary instruments: a micro-computed tomographic study. J Endod 2014;40(11):1860–1864. DOI: 10.1016/j.joen.2014.06.023.
7. Bürklein S, Benten S, Schäfer E. Quantitative evaluation of apically extruded debris with different single-file systems: Reciproc, F360, and OneShape versus Mtwo. Int Endod J 2014;47(5):405–409. DOI: 10.1111/iej.12161.
8. Bürklein S, Mathey D, Schäfer E. Shaping ability of ProTaper NEXT and BT-RaCe nickel-titanium instruments in severely curved root canals. Int Endod J 2015;48(8):774–781. DOI: 10.1111/iej.12375.
9. Pasqualini D, Alovisi M, Cemenasco A, et al. Micro-computed tomography evaluation of ProTaper next and biorace shaping outcomes in maxillary first molar curved canals. J Endod 2015;41(10):1706–1710. DOI: 10.1016/j.joen.2015.07.002.
10. Lopes RMV, Marins FC, Belladonna FG, et al. Untouched canal areas and debris accumulation after root canal preparation with rotary and adaptive systems. Austr Endod J 2018;44(3):260–266. DOI: 10.1111/aej.12237.
11. Linden D, Boone M, De Bruyne M, et al. Adjunctive steps for the removal of hard tissue debris from the anatomic complexities of the mesial root canal system of mandibular molars: a micro-computed tomographic study. J Endod 2020;46(10):1508–1514. DOI: 10.1016/j.joen.2020.05.009.
12. Stringheta CP, Bueno CES, Kato AS, et al. Micro-computed tomographic evaluation of the shaping ability of four instrumentation systems in curved root canals. Int Endod J 2019;52(6):908–916. DOI: 10.1111/iej.13084.
13. Silva EJNL, Vieira VTL, Belladonna FG, et al. Cyclic and Torsional Fatigue Resistance of XP-endo Shaper and TRUShape Instruments. J Endod 2018;44(1):168–172. DOI: 10.1016/j.joen.2017.08.033.
14. Azim AA, Piasecki L, da Silva Neto UX, et al. XP shaper, a novel adaptive core rotary instrument: micro-computed tomographic analysis of its shaping abilities. J Endod 2017;43(9):1532–1538. DOI: 10.1016/j.joen.2017.04.022.
15. živkoviĆ S, NeškoviĆ J, JovanoviĆ-MedojeviĆ M, et al. The efficacy of XP-endo SHAPER (XPS) in cleaning the apical third of the root canal. Serbian Dent J 2017;64(4):171–178. DOI: 10.1515/sdj-2017-0016.
16. Elnaghy AM, Mandorah A, Elsaka SE. Effectiveness of XP-endo Finisher, EndoActivator, and File agitation on debris and smear layer removal in curved root canals: a comparative study. Odontology 2017;105(2):178–183. DOI: 10.1007/s10266-016-0251-8.
17. Zhao Y, Fan W, Xu T, et al. Evaluation of several instrumentation techniques and irrigation methods on the percentage of untouched canal wall and accumulated dentine debris in C-shaped canals. Int Endod J 2019;52(9):1354–1365. DOI: 10.1111/iej.13119.
18. Leoni GB, Versiani MA, Silva-Sousa YT, et al. Ex vivo evaluation of four final irrigation protocols on the removal of hard-tissue debris from the mesial root canal system of mandibular first molars. Int Endod J 2017;50(4):398–406. DOI: 10.1111/iej.12630.
19. Jayakumaar A, Ganesh A, Kalaiselvam R, et al. Evaluation of debris and smear layer removal with XP-endo finisher: a scanning electron microscopic study. Indian J Dent Res 2019;30(3):420–423. DOI: 10.4103/ijdr.IJDR_655_17.
20. Alakshar A, Saleh ARM, Gorduysus MO. Debris and smear layer removal from oval root canals comparing XP-Endo Finisher, EndoActivator, and manual irrigation: a SEM evaluation. Eur J Dent 2020;14(4):626–633. DOI: 10.1055/s-0040-1714762.
21. Alves FRF, Andrade-Junior CV, Marcelino-Alves MF, et al. Adjunctive steps for disinfection of the mandibular molar root canal system: a correlative bacteriologic, micro-computed tomography, and cryopulverization approach. J Endod 2016;42(11):1667–1672. DOI: 10.1016/j.joen.2016.08.003.
22. Azim AA, Aksel H, Zhuang T, et al. Efficacy of 4 irrigation protocols in killing bacteria colonized in dentinal tubules examined by a novel confocal laser scanning microscope analysis. J Endod 2016;42(6):928–934. DOI: 10.1016/j.joen.2016.03.009.
23. Silva EJNL, Belladonna FG, Zuolo AS, et al. Effectiveness of XP-endo Finisher and XP-endo Finisher R in removing root filling remnants: a micro-CT study. Int Endod J 2018;51(1):86–91. DOI: 10.1111/iej.12788.
24. Wigler R, Dvir R, Weisman A, et al. Efficacy of XP-endo finisher files in the removal of calcium hydroxide paste from artificial standardized grooves in the apical third of oval root canals. Int Endod J 2017;50(7):700–705. DOI: 10.1111/iej.12668.
25. Vertucci FJ. Root canal anatomy of the human permanent teeth. Oral Surg Oral Med Oral Pathol 1984;58(5):589–599. DOI: 10.1016/0030-4220(84)90085-9.
26. Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol 1971;32(2):271–275. DOI: 10.1016/0030-4220(71)90230-1.
27. Paqué F, Laib A, Gautschi H, et al. Hard tissue debris accumulation analysis by high-resolution computed tomography scans. J Endod 2009;35(7):1044–1047. DOI: 10.1016/j.joen.2009.04.026.
28. Robinson JP, Lumley PJ, Claridge E, et al. An analytical Micro CT methodology for quantifying inorganic dentine debris following internal tooth preparation. J Dent 2012;40(11):999–1005. DOI: 10.1016/j.jdent.2012.08.007.
29. De-Deus G, Marins J, de Neves A, et al. Assessing accumulated hard-tissue debris using micro-computed tomography and free software for image processing and analysis. J Endod 2014;40(2):271–276. DOI: 10.1016/j.joen.2013.07.025.
30. Versiani MA, Alves FRF, Andrade-Junior CV, et al. Micro-CT evaluation of the efficacy of hard-tissue removal from the root canal and isthmus area by positive and negative pressure irrigation systems. Int Endod J 2016;49(11):1079–87. DOI: 10.1111/iej.12559.
31. Rubin LM, Skobe Z, Krakow AA, et al. The effect of instrumentation and flushing of freshly extracted teeth in endodontic therapy: a scanning electron microscope study. J Endod 1979;5(11):328–335. DOI: 10.1016/S0099-2399(79)80088-6.
32. de Oliveira MAVC, Venâncio JF, Pereira AG, et al. Critical instrumentation area: influence of root canal anatomy on the endodontic preparation. Braz Dent J 2014;25(3):232–236.
33. FKG Dentaire SA. XP-Endo shaper: the one to shape your success; n.d. Available at: http://www.fkg.ch/sites/default/files/201704_fkg_xp_endo_shaper_brochure_v4_en_web.pdf. [Accessed July 7, 2017].
34. Zehnder M. Root canal irrigants. J Endod 2006;32(5):389–98. DOI: 10.1016/j.joen.2005.09.014.
35. Carvalho AS, Camargo CHR, Valera MC, et al. Smear layer removal by auxiliary chemical substances in biomechanical preparation: a scanning electron microscope study. J Endod 2008;34(11):1396–1400. DOI: 10.1016/j.joen.2008.08.012.
36. Gu L, Wei X, Ling J, et al. A microcomputed tomographic study of canal isthmuses in the mesial root of mandibular first molars in a Chinese population. J Endod 2009;35(3):353–356. DOI: 10.1016/j.joen.2008.11.029.
37. Keleş A, Alçin H, Sousa-Neto MD, et al. Supplementary steps for removing hard tissue debris from isthmus-containing canal systems. J Endod 2016;42(11):1677–1682. DOI: 10.1016/j.joen.2016.07.025.
38. Yang Q, Liu MW, Zhu LX, et al. Micro-CT study on the removal of accumulated hard-tissue debris from the root canal system of mandibular molars when using a novel laser-activated irrigation approach. Int Endod J 2020;53(4):529–538. DOI: 10.1111/iej.13250.
39. Uzunoglu-özyürek E, Karaaslan H, Türker SA, et al. Influence of size and insertion depth of irrigation needle on debris extrusion and sealer penetration. Restor Dent Endod 2017;43(1):1e2. DOI: 10.5395/rde.2018.43.e2.
40. Weiger R, ElAyouti A, Löst C. Efficiency of hand and rotary instruments in shaping oval root canals. J Endod 2002;28(8):580–583. DOI: 10.1097/00004770-200208000-00004.
41. Paqué F, Balmer M, Attin T, et al. Preparation of oval-shaped root canals in mandibular molars using nickel-titanium rotary instruments: a micro-computed tomography study. J Endod 2010;36(4):703–707. DOI: 10.1016/j.joen.2009.12.020.
________________________
© The Author(s). 2020 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.